Isomyosamine
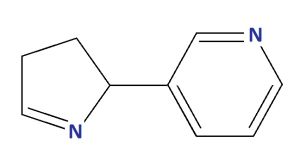
| Isomyosamine |
|---|
| 3-(3,4-DIHYDRO-2H-PYRROL-2-YL)PYRIDINE |
| Molecular Formula C9H10N2 |
| Molecular Weight 146.19 |
| CAS 53844-46-5 |
| PUBCHEM 11286546 |
| brand name MYMD-1® |
Isomyosamine (isomyosmine), an isomer of myosmine, known by the brand name MYMD-1®, is a synthetic alkaloid derived from tobacco plant with potential lifespan extending properties.[1]
Isomyosamine is capable of suppressing production of tumor necrosis factor alpha (TNF-α): an immune cell signalling protein and inflammatory cytokine responsible for inducing and maintaining the inflammatory process.[2][3][4] TNF-α is located upstream of a cascade of molecular signals that induces inflammation, promotes insulin resistance and helps activate the process of aging.[5] Many in vivo and in vitro studies showed that TNFα plays a causative role in the pathogenesis of various age-related diseases, including diabetes,[6][7] Alzheimer’s disease,[8][9] rheumatoid arthritis.[10]
MyMD-1 targets the root causes of inflammation and regulates the immuno-metabolic system through the modulation of numerous pro-inflammatory cytokines, including TNF-α, IL-6 and IL-17A.[3][11] Moreover, isomyosamine exhibits similar biological activities to mTOR inhibitors rapamycin, everolimus and sirolimus owing to their largely overlapping mechanisms of action.[1] Furthermore, MyMD-1 even slightly outperformed rapamycin in a mouse longevity study.[1]

Rapamycin is the gold standard longevity drug, given its consistency and reproducibility in extending lifespan and prolonging healthspan across a wide spectrum of animal models.[12] The non-inferiority study of MyMD-1 when compared to rapamycin, make it an attractive alternative, especially if MyMD-1 superiority proves to be reproducible, as claimed by the study in C57BL/6 mice.[1] For now, it is still premature to conclude MyMD-1 is superior to rapamycin and further studies will need to replicate these findings, potentially via the NIH Aging Interventions Testing Program (ITP).
Apart from longevity, isomyosamine holds significant therapeutic potential in many autoimmune conditions such as autoimmune thyroiditis,[3] multiple sclerosis,[13][14] autoimmune encephalomyelitis,[11] and rheumatoid arthritis.[13][15]
The ease of Isomyosamine oral dosing is a groundbreaking differentiator compared to currently available TNF-α blockers, all of which require delivery by injection or infusion, whereas MyMD-1 is available orally as capsules.[13] MyMD-1 is not detrimental to cell viability and is a small molecule that can cross the blood-brain barrier, potentially enabling it to treat brain-related disease. Commercial efforts are currently evaluating the effectiveness of MyMD-1 in Phase II studies for treating sarcopenia/frailty[16] as a result of the aging process, and other early-stage clinical trials are evaluating its use for rheumatoid arthritis (RA), with the potential to expand into other applications. (see: NCT05283486)
References
- ↑ 1.0 1.1 1.2 1.3 Sabini, E., O’Mahony, A., & Caturegli, P. (2022). MyMD-1 Improves Health Span and Prolongs Life Span in Old Mice: A Noninferiority Study to Rapamycin. The Journals of Gerontology: Series A. PMID:35914953 DOI:10.1093/gerona/glac142
- ↑ Kaplin, B. A. (2021). Eliciting the Hormetic Effect: The Importance of Plant Alkaloids for Treatment of Inflammation. Industry Insight Published: February 15, 2021
- ↑ 3.0 3.1 3.2 Di Dalmazi, G., Chalan, P., & Caturegli, P. (2019). MYMD-1, a novel immunometabolic regulator, ameliorates autoimmune thyroiditis via suppression of Th1 responses and TNF-α release. The Journal of Immunology, 202(5), 1350-1362. PMID:30674573 DOI:10.4049/jimmunol.1801238
- ↑ Eckford C., Brager J. (2023). Developing a first-in-class small molecule drug for inflammatory disease. European Pharmaceutical Review.
- ↑ Sethi, J. K., & Hotamisligil, G. S. (2021). Metabolic Messengers: tumour necrosis factor. Nature metabolism, 3(10), 1302-1312. PMID:34650277 DOI: 10.1038/s42255-021-00470-z
- ↑ Akash, M. S. H., Rehman, K., & Liaqat, A. (2018). Tumor necrosis factor‐alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. Journal of cellular biochemistry, 119(1), 105-110. PMID: 28569437 DOI: 10.1002/jcb.26174
- ↑ Alzamil, H. (2020). Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. Journal of obesity, 2020. PMID: 32089876 PMCID: PMC7013317 DOI: 10.1155/2020/5076858
- ↑ Decourt, B., K Lahiri, D., & N Sabbagh, M. (2017). Targeting tumor necrosis factor alpha for Alzheimer’s disease. Current Alzheimer Research, 14(4), 412-425. PMID: 27697064 PMCID: PMC5328927 DOI: 10.2174/1567205013666160930110551
- ↑ Torres-Acosta, N., O’Keefe, J. H., O’Keefe, E. L., Isaacson, R., & Small, G. (2020). Therapeutic potential of TNF-α inhibition for Alzheimer’s disease prevention. Journal of Alzheimer's Disease, 78(2), 619-626. PMID: 33016914 PMCID: PMC7739965 DOI: 10.3233/JAD-200711
- ↑ BRENNAN, F. M., Maini, R. N., & Feldmann, M. (1992). TNFα—a pivotal role in rheumatoid arthritis?. Rheumatology, 31(5), 293-298. PMID: 1581770 DOI: 10.1093/rheumatology/31.5.293
- ↑ 11.0 11.1 Glenn, J. D., Pantoja, I. M., Caturegli, P., & Whartenby, K. A. (2020). MYMD-1, a novel alkaloid compound, ameliorates the course of experimental autoimmune encephalomyelitis. Journal of Neuroimmunology, 339, 577115. PMID:31778849 DOI:10.1016/j.jneuroim.2019.577115
- ↑ Fontana, L., Partridge, L., & Longo, V. D. (2010). Extending healthy life span—from yeast to humans. science, 328(5976), 321-326.
- ↑ 13.0 13.1 13.2 Brager, J., Chapman, C., Dunn, L., & Kaplin, A. (2023). A Double-blind, Placebo-controlled, Randomized, Single Ascending, and Multiple Dose Phase 1 Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Oral Dose Isomyosamine Capsules in Healthy Adult Subjects. Drug Research, 73(02), 95-104. PMID: 36368677 PMC9902179 DOI: 10.1055/a-1962-6834
- ↑ MyMD-1: Potential treatment for s Multiple Sclerosis Depression
- ↑ MyMD Pharmaceuticals® Announces Upcoming Presentation of Preclinical Rheumatoid Arthritis Data for Oral TNF-α Inhibitor MYMD-1® at the Society of Toxicology 2023 Annual Meeting on March 20, 2023, at 9:00 AM CT
- ↑ The Phase 2 multi-center double-blind, placebo controlled, randomized study (NCT05283486) investigates the efficacy, tolerability and pharmacokinetics of MYMD-1 in the treatment of chronic inflammation associated with sarcopenia/frailty in participants aged 65 years or older.]