FAQ
Longevity biotechnology is a new field of medical research that aims delay, prevent or reverse age-related diseases to extend healthy human lifespan. This article provides an overview of the field through answering frequently asked questions.
Why is aging a problem?


Aging is the largest risk factor for the diseases that kill most people worldwide, including cancer, heart diseases and Alzheimer's disease.[2]
In the US in 2020, 9 of the 10 leading causes of death were strongly age-related.[3] Globally, approximately 70% of deaths annually are the result of aging.[4]
Aging substantially reduces quality of life. Deaths due to aging are usually preceded by many years of diseases such as arthritis, type 2 diabetes and Alzheimer’s, leading to loss of function and independence.
As a result of the aging process, the risk of an individual dying increases exponentially, notably after around 30 years old.[5] This increase in mortality rate is known as the Gompertz-Makeham law of mortality.[1][5]
How many people die from aging per day?
Aging and its related diseases result in the deaths of 100,000 people per day; more than twice the sum of all other causes of death combined.[4] This equates to over 37 million people dying per year of aging - a population the size of Canada. This is because aging results in the exponential increasing risk of age-related diseases such as cancer and type 2 diabetes. Aging also accounts for more than 30% of all disability-adjusted life years lost (DALYs); more than any other single cause.[6]

Can healthy lifespan be extended?

Clinical trials for longevity drugs in humans are in-progress, and there is not yet evidence that drugs can slow aging in humans. However, recent experimental breakthroughs over the last few decades have shown that in mice, worms and flies, healthy lifespan can be modified.[8] Many clinical trials in humans today are testing whether these results can be replicated in humans.[9]
In animal models, slowing the aging process in animals can be achieved via manipulation of diet, environment, genetics, epigenetics, or with drugs. In mice, over 100 drugs have been shown to extend healthy lifespan by up to 30%.[7][8]
The effect of longevity drugs on mice is often significant, delaying or eliminating the burden of age-related problems such as frailty, cataracts, cancer, and sarcopenia. For example, a new class of drugs called senolytics have been shown to extend healthy lifespan in mice by over 30% whilst delaying age-related dysfunction.
Which therapies may extend healthy lifespan?
Several drugs are known to extend healthy lifespan in animals. While there is no conclusive data that suggests these drugs would work the same in humans, many of these drugs are now being tested in clinical trials.

Senolytics
Senolytics are drugs that remove senescent cells. These are cells which accumulate in the body with age and are linked with age-related diseases such as cancer.[11]

In mice, senolytics have been shown to increase the healthy lifespan by up to 35%.[10] The first clinical trials of senolytics in humans began in 2016, demonstrating improved inflammation biomarkers and function, suggestive of benefit in humans.[13][14]
Metformin
Metformin is an approved drug for type 2 diabetes that extends lifespan in multiple species. In mice, Metformin extends lifespan by up to 6%.[15] Metformin works in part by improving insulin sensitivity and may target several hallmarks of aging.[12] Metformin is being tested as a longevity therapy in the largest clinical trial of its kind, the Targeting Aging with Metformin (TAME) trial in the US.[16] This is a randomized clinical trial of 3000 elderly patients comparing metformin against placebo for the onset of age-related diseases.[16] Whether it slows aging in humans is unproven; the TAME trial may provide some insight.
Rapamycin

Rapamycin, also known as Rapamune©, is a drug used to prevent the rejection of organ transplants by the immune system. Rapamycin has been shown to extend lifespan by up to 26% when given at middle age, and 14% when given in late life.[18][19]
Among the hundreds of interventions known to target aging, rapamycin is unique in its highly consistent and reproducible effect on healthy lifespan extension in mice.[20][21]
Rapamycin is being tested in a large-scale clinical trial called the Participatory Evaluation (of) Aging (with) Rapamycin (for) Longevity Study (PEARL). This is a randomized clinical trial aiming to enroll 1000 elderly patients comparing rapamycin against placebo for age-related outcomes.[17]
Epigenetic reprogramming
Epigenetic reprogramming refers to remodeling of epigenetic marks, such as methylation tags on the DNA within cells. A specific version of this technique was used in 2020 to restore vision to blind mice by fully regrowing the optic nerve.[22] The lead researcher David Sinclair at Harvard Medical School believes this approach could one day be used to regenerate other tissues of the body as a longevity strategy.[23]
In addition, over 50 other drugs are being tested in human clinical trials for targeting aging, to prevent or reverse various diseases.[24]
What is the goal of longevity biotechnology?

Longevity biotechnology aims to slow or reverse the aging process to extend the healthy lifespan (‘healthspan’) of the population. Instead of treating diseases when they arise, one by one, longevity biotechnology aims to keep people healthy by slowing the aging process. The aim is to thereby prevent or reverse multiple age-related diseases at once.
The longevity biotechnology sector is rapidly growing, and there are currently over 170 private companies working on developing therapeutics to slow the aging process.[26] These companies are working on slowing or reversing the aging process by targeting one or more of the hallmarks of aging.
Will healthy or unhealthy lifespan be extended?

The stated goal of longevity biotechnology is to extend the healthy period of lifespan, particularly before age-related diseases set in. This is in contrast to many of today's medical interventions, which extend life only after diseases (e.g. cancer, Alzheimer's) have reached a clinical level.
Age-related diseases are preceded by a long period of subclinical aging, i.e. aging that has not yet progressed far enough to produce clinical symptoms. By slowing, delaying or reversing the aging process, the hypothesis is that the healthy period of life can be extended.[28]
Studies in mice have demonstrated that life extending drugs such as rapamycin are capable of extending the healthy, rather than unhealthy period of life.[18]
Should we treat specific diseases such as cancer, or focus on aging?
![Slowing aging is potentially a more effective means of extending healthy lifespan than treating individual diseases due to the comorbid nature of age-related diseases. [6]](https://static.longevitywiki.org/en/thumb/Slowing_aging_versus_curing_cancer.jpg/421px-Slowing_aging_versus_curing_cancer.jpg)
Longevity biotechnology offers a potentially greater likelihood of extending healthy lifespan than the current approach - attempting to find cures for the individual diseases of aging. This is because biological aging is associated with many diseases of aging. These diseases develop as comorbidities, which occur in sequence. Even if a person survives one age-related disease such as cancer, another (e.g. dementia, heart disease) is likely to next kill the person, because the underlying aging process is not treated.
This phenomenon is known as the Taeuber paradox[30], and accounts for the much smaller projected increase in healthy lifespan associated with curing the diseases of aging, such as cancer (2-3 years), versus slowing aging itself (30+ years).[29][30][31] The life expectancy estimate of the latter is based on calculations by Professor Kaeberlein at the University of Washington, using lifespan data from rapamycin-treated mice.[32]
What is biological aging?
There are two main types of 'age': chronological age - the number of years since birth, and biological age - a measure of the physical health of a person and their position in their lifespan. Biological age is more difficult to measure, but recent technologies are now available to estimate biological age. Over time, we age biologically. This occurs due to many processes in the body, which are generally categorized into 9 processes or 'hallmarks'.[2]
What are the 9 Hallmarks of Aging?

The hallmarks of aging framework is considered to broadly represent key biological mechanisms of the biological aging process.[2] Although there are limitations of the hallmarks framework, it has become the central framework for understanding aging biology. The nine forms of cellular and molecular ‘damage’ that comprise the hallmarks of aging are[2]:
- Genomic instability
- Telomere attrition
- Epigenetic alterations
- Loss of proteostasis
- Deregulated nutrient-sensing
- Mitochondrial dysfunction
- Cellular senescence
- Stem cell exhaustion
- Altered intercellular communication
These are thought to be the lowest common denominators of the aging process, on a cellular or subcellular level. All of the physiological changes associated with aging, such as greying hair, wrinkles, frailty and an increased risk of disease are thought to stem from these underlying processes.[2]
How does the aging cause disease?
The nine hallmarks of aging have been shown to play an integral role in the development of many age-related diseases such as dementia, cancer, and a multitude of others:
Neurodegenerative disease

All of the hallmarks of aging are associated with an increased risk of neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease.[34] For example, several types of DNA damage are associated with neurodegeneration. The shortening of telomeres occurs as part of biological aging and causes cellular senescence, and is associated with neurodegeneration and neurodegenerative diseases including Alzheimer's disease and Parkinson's disease.[33] Additionally, mitochondrial dysfunction, altered metabolism, stem cell exhaustion and loss of proteostasis are also involved in the development of neurodegenerative diseases.[33]
Cancer
Several of the hallmarks of aging such as cellular senescence and alterations in the extracellular matrix are responsible for increasing the likelihood of tumorigenesis.[35]
Other hallmarks of aging such as genomic instability (DNA mutations), loss of proteostasis (e.g. proteotoxic stress), altered intercellular communication (e.g. inflammation) and epigenetic alterations (e.g. aberrant DNA methylation) are also involved in cancer development.[35]
How is biological age measured?

Recent technologies have helped the measure of biological age, including:
Epigenetic clocks
The first generation of aging clocks are known as epigenetic clocks or Horvath’s clock.[36] This clock is based on the finding that with aging, the body accumulates methylation tags on the DNA in a pattern that can be predicted with machine learning. These changes to the epigenome are influenced by lifestyle and environment, and can be used to measure biological age.[36] Factors such as exercise frequency and a low BMI have been shown to reduce the rate of epigenetic aging, whereas obesity and smoking can accelerate this rate.[37][38][39] Horvath's clock has been used to accurately predict mortality risk among human populations.[40]
Multi-omic clocks
Epigenetic clocks are being developed that incorporate data from several sources. This may include multi-omic clocks which include metabolomics, proteomics, or non-molecular biomarkers such as body imaging and fitness tests.[41] These clocks are built using machine learning to estimate biological age. These aging clocks are being developed that combine multiple biological functional tests to create a unique aging signature, which can be monitored over time.[41]

Which companies are trying to solve aging?

There are over 170 longevity biotechnology companies trying to create therapies to slow or reverse the aging process.[26] There are over 50 longevity drugs currently in clinical trials in humans.[24]
Many of the longevity biotechnology companies are targeting specific hallmarks of aging. For example, Cleara Biotech are attempting to reduce cellular senescence by developing a drug that can eliminate senescent cells.[43]
Calico Labs is a Google-backed biotech company with the goal of combating aging and age-related diseases. In 2014, the company created a partnership with pharmaceutical giant AbbVie, which has since developed into a $2.5 billion venture in the pursuit of improving “health, wellbeing and longevity.” [42]
Since aging is not currently considered a disease by regulatory bodies such as the Food and Drug Administration (FDA), drug developers are currently focused on specific diseases of aging such as glaucoma and osteoarthritis.[44]
Which billionaires are funding longevity biotechnology?

Several billionaires have funded companies or initiatives to slow or reverse human aging. These include:
- Jeff Bezos, co-founder of Amazon, helped raise $3 billion for new anti-aging drug company Altos Labs in 2021.[45]
- Sam Altman, CEO of OpenAI, invested $180 million into RetroBiosciences whose goal is to add 10 years to healthy human lifespan.[46]
- Peter Thiel, co-founder of PayPal, was an early investor in Unity Biotechnology.[47]
- Sergey Brin, co-founder of Google, donated $25 million for the National Academy of Medicine’s Grand Challenge in Health Longevity to 'end aging forever'.[48]
- Larry Page, co-founder of Google, co-founded the billion-dollar aging research company Calico Labs.[49]
- Mike Cannon-Brookes, billionaire cofounder of Australian software giant Atlassian, has invested $10 million into longevity company Juvenescence.[50]
- Jim Mellon, UK billionaire investor who co-founded longevity company Juvenescence.[51]
- Larry Ellison, founder of Oracle, has spent $430 million on longevity research.[52]
- Michael Greve, founder of Kizoo Technology, who has pledged €300m to rejuvenation biotechnology companies.[53]
- Naveen Jain, billionaire entrepreneur who has raised $54 million for his startup Viome.[54]
- Yuri Milner, billionaire tech investor who helped Altos Labs raise $3 billion, with Jeff Bezos.[45]
- Brian Armstrong, CEO of CoinBase, who helped found and raise $105 million for the epigenetic reprogramming startup NewLimit.[55]
- Vitalik Buterin, founder of the cryptocurrency Ethereum, who has donated over $2.4 million to anti-aging research organization SENS, among various other biotech projects.[56]
How large will the longevity biotechnology field be?
Analysts from the Bank of America have predicted that the market size of longevity biotechnology will reach $600 billion by 2025.[57] Others have speculated that it is a trillion dollar industry owing to the immense savings associated with delaying or preventing chronic diseases.[58]
Is age an important for risk factor for COVID-19 mortality?
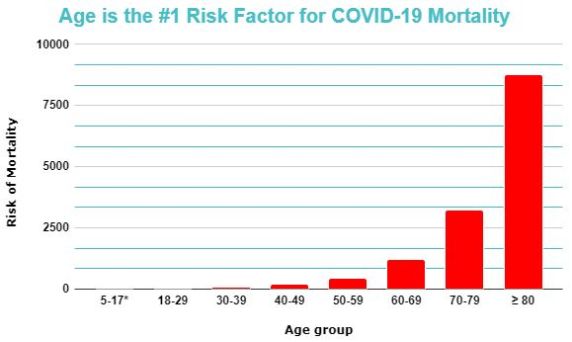
Age is the single most significant risk factor for COVID-19 mortality. The mortality rate from COVID-19 increases exponentially with age, such that the death rate for those aged over 80 years is over 1000 times higher than those below 30 years.[59]
This increase in COVID-19 mortality with age is thought to be the result of a weakening of the immune system with age, known as immunosenescence.[60]
The mortality rate doubling time for COVID-19 is close to the all-cause mortality rate doubling time.[59] This has led several scientists to conclude that COVID-19 meets criteria for an age-related disease.[59][61][62][63][64][65]
How is aging biology research different to other fields of medical research?
Scientists studying the biology of aging believe that aging is a root cause of all the major diseases of aging, and that targeting aging directly would treat or reverse multiple diseases, simultaneously. This is known as the geroscience hypothesis, and has garnered traction in recent years.[28]
The approach of targeting aging directly differs from many fields in mainstream medical research such as cancer research, which seek to find cures for individual diseases. An argument in favour of targeting aging directly is that targeting single diseases leads to diminishing returns in healthy lifespan extension.[29] Due to the many diseases that occur concurrently in older age, completely curing a single disease such as cancer would only add 2-3 healthy years of life on average, whereas slowing aging could add 30 or more healthy years.[29] This is because another disease in line, e.g. Alzheimer's or lung disease, will subsequently result in death, a phenomenon known as the Taeuber Paradox.[66]
Do all animals age in the same way?

Many species, due to differences in their biology, age at different rates to humans. The jellyfish Turritopsis dohrnii can revert to earlier stages of its life cycle in response to stress, and is theoretically biologically immortal - though eventually dies, usually due to predation.[68]
Among mammals, the naked mole rat is an exceptionally long lived organism. It lives roughly ten times longer than similarly-sized rats, with resistance to cancer and age-related diseases.[69][70] Unlike other organisms, such as humans, horses and mice, the mortality rate of the naked mole rat appears steady over time. This trend does not follow the exponential increase in mortality (Gompertz-Makeham law) that humans do.[69]
Scientists are now studying the naked mole rat to identify key patterns in their genetics, environmental traits, and metabolism that may be responsible for their longer lifespans.[71]

Is aging considered a disease?
Aging is not currently classified as a disease by regulatory bodies such as the Food and Drug Administration (FDA) in the United States. However, several scientists in the aging field have publicly stated their preference for aging to be defined as, or at least thought of, as a disease, including professors David Sinclair and Nir Barzilai.[72]
Recognition of aging as a treatable medical condition by the WHO comes from the new ICD-11 extension code MG2A and XT9T. MG2A refers to “Ageing associated decline in intrinsic capacity”, and is classified under "general symptoms".[73] XT9T refers to "Ageing-related”, under the "Causality" category, and recognizes aging as a contributor to disease.[74]
What are the economic benefits of extending healthy lifespan?
Extending the healthy human lifespan could have significant economic benefits gobally. An economic analysis from 2021 by researchers at the University of Oxford and Harvard University estimated the benefit of a drug that slows aging by 1 year as $38 trillion.[75] This is mainly because slowing aging prevents age-related diseases such as cancer and Alzheimer’s disease, which are of great expense to healthcare systems, productivity, and society. The study demonstrated a larger economic benefit of slowing aging than curing the individual diseases of aging. [75]
Which scientists and institutions are trying to understand and reverse aging?
Over 300 scientists are working on understanding the biology of aging in leading institutions that include Harvard University, Stanford University, Yale University, and the University of Oxford.[76] Some of the most well-known institutes and labs include:
The Buck Institute for Research on Aging
The Buck Institute for Research on Aging is one of the largest longevity research institutes with over 250 scientists. The research covers several areas including the mechanisms of aging, neurodegeneration, senescence, stem cells and regenerative medicine, cellular stress and disease, cancer associated with aging, and mitochondrial function.[77]
Professor David Sinclair - Harvard Medical School
Professor Sinclair’s lab focuses on understanding and reversing aging. The lab focuses on a range of areas including DNA repair, mitochondrial dysfunction, and the interactions between epigenetic and genetic instability, and tissue reprogramming.[78]
Professor Matt Kaeberlein - University of Washington
Professor Kaeberlein's lab focuses on biological mechanisms of aging in order to facilitate translational interventions that promote healthspan and improve quality of life. Kaeberlein is known for his work on the longevity drug rapamycin in organisms such as mice and dogs. He is Director of the Dog Aging Project, a multi-year initiative studying the genetic and environmental factors that influence health, with over 33,000 participating dogs.[79]
Professor Brian Kennedy - National University of Singapore
Professor Kennedy’s lab focuses on understanding the biology of aging and translating research discoveries into new ways of delaying aging in humans. The lab has identified drugs that extend the healthy lifespan of worms and mice and are seeking to understand their mechanisms.[80]
Associate Professor Lynne Cox - University of Oxford
Professor Cox’s lab study the molecular and cellular basis of aging to identify specific biochemical processes and pathways that change organisms age. The lab particularly cellular senescence, which underpins many age-related diseases including cancer and neurodegeneration.[81]
What books have been written on this topic?
Several books have been written on the topic of aging and longevity. These include:
Which anti-aging drugs are being tested in clinical trials today?
There are over 50 drugs being tested in human clinical trials for aging or age-related diseases.
The largest trial is the Targeting Aging with Metformin (TAME) trial, which began in 2020. The trial is being run in the United States with a cohort of 3000 older adults. The goal of the TAME trial is to determine whether diabetes drug metformin slows the aging process in older adults. The TAME trial is a $75 million trial run by the American Federation for Aging Research.[16]
Another large clinical trial is the Participitory Evaluation (of) Aging (with) Rapamycin (for) Longevity (PEARL) study. The goal of this study is to determine whether rapamycin, a drug currently approved for immunosuppression during organ transplants, slows biomarkers of aging in 1000 adults. The PEARL trial is being run by AgelessRx.[17]
Is aging a natural process that should be accepted?
Although aging is a natural process in humans, it is the driving force of many diseases such as cancer and Alzheimer's disease - which as a society we have decided are worth trying to find cures for. In the past, these conditions, as well as atherosclerosis and even infectious diseases were once thought of as natural processes to be accepted. As treatments for previously untreatable diseases started being developed, society moved to accommodate this.
Atherosclerosis, the hardening of the arteries, was once viewed as a natural process that was simply a consequence of aging. However, when treatments such as statins were later developed to prevent atherosclerosis, it became widely regarded as a disease to be cured. Statins are now one one of the most widely prescribed drugs in the world, used in preventing heart diseases. The inventor of statins, Akira Endo, describes in his historical paper: “Serious research on the role of cholesterol in human atherosclerosis did not really get underway until the 1940s, due to a prevailing view that the disease was a simple consequence of aging and could not be prevented.”[82]
Will anti-aging technology lead to overpopulation?
A common objection to the development of longevity technologies is that they may lead to an unsustainably large population size.
One study of a 2005 Swedish cohort of 9 million people modeled the effect of various anti-aging scenarios. The researchers showed that even with the most radical life-extension technology in which aging completely stopped after age 60, the population growth across 100 years was only 22%.[83] In reality, the first aging drugs will likely slow aging by a modest amount, and most likely increase healthspan as opposed to lifespan. This is partly based on evidence in animals showing that it is easier to extend median, as opposed to maximal lifespan.
Currently, the world’s population is 7.9 billion, and the WHO predicts the population will peak at 11 billion in 2100 before falling due to declining birth rates in many parts of the world. The fastest growing populations are in Africa and South-east Asia. However, several factors are thought to reduce birth rates over time, including increased access to contraceptives, female empowerment, increased education and increased employment. There is evidence that populations that move to a more advanced economy show a reduction in birth rates. This is expected to mitigate the increase in population size from longer lifespans.
Will anti-aging drugs only be available to the rich?
It is unclear who will have first access to longevity drugs. Several researchers in the field have argued that several economic factors are likely to drive down the price of drugs that slow aging.
- The cost of many biotechnologies decreases substantially over time. For example, the first human genome cost $100 million to sequence, and is now available for a few hundred dollars.[84]
- The price of many lifesaving pharmaceuticals has fallen significantly in price once generic drugs were produced. For example, Lipitor, a statin used to prevent heart disease has fallen in price from $85 in 2011 to less than $5 today.
- Many companies in the longevity field have stated that equity of access is part of their core ethos, and many researchers are part of an international coalition called the Academy of Health & Lifespan research which aims to ensure that breakthroughs in aging research are accessible to all. [85]
References
- ↑ 1.0 1.1 En.wikipedia.org. 2021. Gompertz–Makeham law of mortality - Wikipedia. [online] Available at: https://en.wikipedia.org/wiki/Gompertz%E2%80%93Makeham_law_of_mortality [Accessed 13 December 2021].
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194-217.
- ↑ 3.0 3.1 Ahmad, F. B., & Anderson, R. N. (2021). The leading causes of death in the US for 2020. JAMA, 325(18), 1829-1830.https://jamanetwork.com/journals/jama/fullarticle/2778234
- ↑ 4.0 4.1 Ritchie, H. and Roser, M., 2021. Causes of Death. [online] Our World in Data. Available at: <https://ourworldindata.org/causes-of-death> [Accessed 13 December 2021].
- ↑ 5.0 5.1 Olshansky, S. J., & Carnes, B. A. (1997). Ever since gompertz. Demography, 34(1), 1-15.
- ↑ Vizhub.healthdata.org. 2021. GBD Compare | IHME Viz Hub. [online] Available at: <https://vizhub.healthdata.org/gbd-compare/> [Accessed 13 December 2021].
- ↑ 7.0 7.1 de Magalhaes, J., 2021. DrugAge: Species Detail. [online] Genomics.senescence.info. Available at: <https://genomics.senescence.info/drugs/species_details.php> [Accessed 14 December 2021].
- ↑ 8.0 8.1 Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., ... & Sierra, F. (2014). Geroscience: linking aging to chronic disease. Cell, 159(4), 709-713.
- ↑ Kaeberlein, M. (2017). Translational geroscience: A new paradigm for 21st century medicine. Translational medicine of aging, 1, 1-4.
- ↑ 10.0 10.1 Baker, D. J., Childs, B. G., Durik, M., Wijers, M. E., Sieben, C. J., Zhong, J., ... & Van Deursen, J. M. (2016). Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature, 530(7589), 184-189.
- ↑ Xu, M., Pirtskhalava, T., Farr, J. N., Weigand, B. M., Palmer, A. K., Weivoda, M. M., ... & Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature medicine, 24(8), 1246-1256.
- ↑ 12.0 12.1 Kulkarni, A. S., Gubbi, S., & Barzilai, N. (2020). Benefits of metformin in attenuating the hallmarks of aging. Cell metabolism, 32(1), 15-30.
- ↑ https://clinicaltrials.gov/ct2/show/NCT02874989
- ↑ https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(18)30629-7/fulltext
- ↑ Anisimov, V. N., Berstein, L. M., Popovich, I. G., Zabezhinski, M. A., Egormin, P. A., Piskunova, T. S., Semenchenko, A. V., Tyndyk, M. L., Yurova, M. N., Kovalenko, I. G., & Poroshina, T. E. (2011). If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging, 3(2), 148–157. https://doi.org/10.18632/aging.100273
- ↑ 16.0 16.1 16.2 TAME - Targeting Aging with Metformin - American Federation for Aging Research. (n.d.). American Federation for Aging Research. https://www.afar.org/tame-trial
- ↑ 17.0 17.1 17.2 Clinicaltrials.gov. 2021. Participatory Evaluation (of) Aging (With) Rapamycin (for) Longevity Study - Full Text View - ClinicalTrials.gov. [online] Available at: <https://clinicaltrials.gov/ct2/show/NCT04488601> [Accessed 14 December 2021].
- ↑ 18.0 18.1 Harrison, D., Strong, R., Sharp, Z. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). https://doi.org/10.1038/nature08221
- ↑ Miller, R. A., Harrison, D. E., Astle, C. M., Fernandez, E., Flurkey, K., Han, M., Javors, M. A., Li, X., Nadon, N. L., Nelson, J. F., Pletcher, S., Salmon, A. B., Sharp, Z. D., Van Roekel, S., Winkleman, L., & Strong, R. (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging cell, 13(3), 468–477. https://doi.org/10.1111/acel.12194
- ↑ Johnson, S. C., Martin, G. M., Rabinovitch, P. S., & Kaeberlein, M. (2013). Preserving youth: does rapamycin deliver? Science translational medicine, 5(211), 211fs40.
- ↑ Kaeberlein, M. (2014). Rapamycin and aging: when, for how long, and how much? Journal of genetics and genomics, 41(9), 459.
- ↑ Lu, Y., Brommer, B., Tian, X. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020). https://doi.org/10.1038/s41586-020-2975-4
- ↑ Reversing The Aging Clock With Epigenetic Reprogramming. (n.d.). Pubs - Bio-IT World. https://www.bio-itworld.com/news/2021/01/13/reversing-the-aging-clock-with-epigenetic-reprogramming
- ↑ 24.0 24.1 Lifespan.io. 2021. The Rejuvenation Roadmap | Lifespan.io. [online] Available at: <https://www.lifespan.io/road-maps/the-rejuvenation-roadmap/> [Accessed 13 December 2021].
- ↑ Longevity companies to watch in 2021 - Longevity.Technology. (n.d.). Longevity.Technology. https://www.longevity.technology/longevity-companies-to-watch-in-2021/
- ↑ 26.0 26.1 Pfleger, K., 2021. Aging Companies. [online] Agingbiotech.info. Available at: <https://agingbiotech.info/companies> [Accessed 13 December 2021].
- ↑ How to save Medicare: the anti-aging remedy. (2012, March). https://www.researchgate.net/figure/From-longer-life-span-to-longer-health-span-and-life-span-From-A-to-B-Standard_fig1_230724035.
- ↑ 28.0 28.1 Austad, S. N. (2016). The geroscience hypothesis: is it possible to change the rate of aging?. In Advances in geroscience (pp. 1-36). Springer, Cham.
- ↑ 29.0 29.1 29.2 29.3 Matt Kaeberlein, PhD, It is Time to Embrace 21st-Century Medicine, Public Policy & Aging Report, Volume 29, Issue 4, 2019, Pages 111–115, https://doi.org/10.1093/ppar/prz022
- ↑ 30.0 30.1 Keyfitz, N. (1977). What difference would it make if cancer were eradicated? An examination of the Taeuber paradox. Demography, 14(4), 411-418.
- ↑ Mitra, S. (1978). A short note on the Taeuber paradox. Demography, 15(4), 621-623.
- ↑ https://twitter.com/mkaeberlein/status/1463580145126567936
- ↑ 33.0 33.1 33.2 Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., & Bohr, V. A. (2019). Ageing as a risk factor for neurodegenerative disease. Nature reviews. Neurology, 15(10), 565–581. https://doi.org/10.1038/s41582-019-0244-7
- ↑ Qiu, C., Kivipelto, M., & von Strauss, E. (2009). Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues in clinical neuroscience, 11(2), 111.
- ↑ 35.0 35.1 Fane, M., & Weeraratna, A. T. (2020). How the ageing microenvironment influences tumour progression. Nature Reviews Cancer, 20(2), 89-106.
- ↑ 36.0 36.1 36.2 Horvath, S., & Raj, K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371-384.
- ↑ Quach, A., Levine, M. E., Tanaka, T., Lu, A. T., Chen, B. H., Ferrucci, L., ... & Horvath, S. (2017). Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY), 9(2), 419.
- ↑ Wu, X., Huang, Q., Javed, R., Zhong, J., Gao, H., & Liang, H. (2019). Effect of tobacco smoking on the epigenetic age of human respiratory organs. Clinical Epigenetics, 11(1), 183. https://doi.org/10.1186/s13148-019-0777-z ↑
- ↑ Horvath, S., Erhart, W., Brosch, M., Ammerpohl, O., von Schönfels, W., Ahrens, M., Heits, N., Bell, J. T., Tsai, P.-C., Spector, T. D., Deloukas, P., Siebert, R., Sipos, B., Becker, T., Röcken, C., Schafmayer, C., & Hampe, J. (2014). Obesity accelerates epigenetic aging of human liver. Proceedings of the National Academy of Sciences, 111(43), 15538–15543. https://doi.org/10.1073/pnas.1412759111 ↑
- ↑ Fransquet, P. D., Wrigglesworth, J., Woods, R. L., Ernst, M. E., & Ryan, J. (2019). The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clinical epigenetics, 11(1), 1-17.
- ↑ 41.0 41.1 41.2 Kudryashova, K. S., Burka, K., Kulaga, A. Y., Vorobyeva, N. S., & Kennedy, B. K. (2020). Aging Biomarkers: From Functional Tests to Multi‐Omics Approaches. Proteomics, 20(5-6), 1900408.
- ↑ 42.0 42.1 2021. Google sister company and drug giant chip in another $1 billion to cure age-related diseases. [online] Available at: <https://www.cnbc.com/2018/06/26/alphabet-backed-calico-and-abbvie-chip-in-1-billion-to-cure-aging.html> [Accessed 15 December 2021].
- ↑ Cleara Biotech. 2021. Cleara Biotech. [online] Available at: <https://www.clearabiotech.com/#DiscoveryTimeline> [Accessed 15 December 2021].
- ↑ Clinicaltrialsarena.com. 2021. Unity's Phase II osteoarthritis study of UBX0101 misses primary goal. [online] Available at: <https://www.clinicaltrialsarena.com/news/unity-ubx0101-osteoarthritis/> [Accessed 15 December 2021].
- ↑ 45.0 45.1 45.2 MIT Technology Review. 2021. Meet Altos Labs, Silicon Valley’s latest wild bet on living forever. [online] Available at: <https://www.technologyreview.com/2021/09/04/1034364/altos-labs-silicon-valleys-jeff-bezos-milner-bet-living-forever/> [Accessed 15 December 2021].
- ↑ MIT Technology Review. 2023. Sam Altman invested $180 million into a company trying to delay death. [online] Available at: <https://www.technologyreview.com/2023/03/08/1069523/sam-altman-investment-180-million-retro-biosciences-longevity-death/> [Accessed 09 July 2024].
- ↑ CNBC. 2021. Why Jeff Bezos is backing this Silicon Valley scientist who is working on a cure for aging. [online] Available at: <https://www.cnbc.com/2018/08/29/-jeff-bezos-is-backing-this-scientist-who-is-working-on-a-cure-for-aging.html> [Accessed 15 December 2021].
- ↑ Google’s co-founders and other Silicon Valley billionaires are trying to live forever. (2021). Retrieved 15 December 2021, from https://www.cnbc.com/2017/03/31/google-co-founders-and-silicon-valley-billionaires-try-to-live-forever.html
- ↑ Contributors to Wikimedia projects. (2013, September 19). Calico (company) - Wikipedia. Wikipedia, the free encyclopedia. https://en.wikipedia.org/wiki/Calico_(company)
- ↑ Shead, S. (2019, August 19). Billionaire Backs U.K. Startup Trying To Extend Human Life Spans. Forbes. https://www.forbes.com/sites/samshead/2019/08/19/billionaire-backs-uk-startup-trying-to-extend-human-lifespans/
- ↑ Jim Mellon - Chairman & Co-Founder. (n.d.). Juvenescence - Science of Healthy Aging & Extended Lifespan. https://www.juvlabs.com/people/co-founder/jim-mellon
- ↑ Tullis, P. (2017, March 30). Are You Rich Enough To Live Forever? Town & Country. https://www.townandcountrymag.com/society/money-and-power/a9202324/science-of-longevity/
- ↑ Michael Greve commits €300m for rejuvenation start-ups. (2021, May 6). Longevity.Technology. https://www.longevity.technology/michael-greve-commits-e300m-for-rejuvenation-start-ups/
- ↑ Gut health startup Viome raises $54M to develop cancer diagnostics and sell microbiome kits. (2021, November 10). Geekwire. https://www.geekwire.com/2021/gut-health-startup-viome-raises-54m-develop-cancer-diagnostics-sell-microbiome-kits/
- ↑ Liberatore, S. (2021, December 14). Billionaire launches new start-up to REVERSE the ageing process. Mail Online. https://www.dailymail.co.uk/sciencetech/article-10310475/Billionaire-launches-new-start-hopes-REVERSE-ageing-process.html
- ↑ Foundation, S. R. (n.d.). SENS Research Foundation Receives $2.4 Million Ethereum Donation From Vitalik Buterin. GlobeNewswire News Room. https://www.globenewswire.com/news-release/2018/02/02/1332410/0/en/SENS-Research-Foundation-Receives-2-4-Million-Ethereum-Donation-From-Vitalik-Buterin.html
- ↑ 2021. Human lifespan could soon pass 100 years thanks to medical tech, says BofA. [online] Available at: <https://www.cnbc.com/2019/05/08/techs-next-big-disruption-could-be-delaying-death.html> [Accessed 14 December 2021].
- ↑ Colangelo, M., 2021. AI Will Drive The Multi-Trillion Dollar Longevity Economy. [online] Forbes. Available at: <https://www.forbes.com/sites/cognitiveworld/2019/12/07/ai-will-drive-the-multi-trillion-dollar-longevity-economy/?sh=294766b74965> [Accessed 14 December 2021].
- ↑ 59.0 59.1 59.2 Santesmasses, D., Castro, J. P., Zenin, A. A., Shindyapina, A. V., Gerashchenko, M. V., Zhang, B., ... & Gladyshev, V. N. (2020). COVID‐19 is an emergent disease of aging. Aging cell, 19(10), e13230.
- ↑ Bajaj, V., Gadi, N., Spihlman, A. P., Wu, S. C., Choi, C. H., & Moulton, V. R. (2021). Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections?. Frontiers in Physiology, 11, 1793.
- ↑ Promislow, D. E. (2020). A geroscience perspective on COVID-19 mortality. The Journals of Gerontology: Series A, 75(9), e30-e33.
- ↑ Alberts, S. C., Archie, E. A., Gesquiere, L. R., Altmann, J., Vaupel, J. W., & Christensen, K. (2014). The male-female health-survival paradox: a comparative perspective on sex differences in aging and mortality. In Sociality, hierarchy, health: comparative biodemography: a collection of papers. National Academies Press (US).
- ↑ Sierra, F. (2020). Geroscience and the Coronavirus Pandemic: The Whack‐a‐Mole Approach is not Enough. Journal of the American Geriatrics Society, 68(5), 951.
- ↑ Barzilai, N., Appleby, J. C., Austad, S. N., Cuervo, A. M., Kaeberlein, M., Gonzalez-Billault, C., ... & Sierra, F. (2020). Geroscience in the Age of COVID-19. Aging and disease, 11(4), 725.
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7288963/
- ↑ En.wikipedia.org. 2021. Taeuber Paradox - Wikipedia. [online] Available at: <https://en.wikipedia.org/wiki/Taeuber_Paradox> [Accessed 14 December 2021].
- ↑ Ruby, J. G., Smith, M., & Buffenstein, R. (2018). Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. elife, 7, e31157.
- ↑ Bavestrello, Giorgio; Christian Sommer; Michele Sarà (1992). "Bi-directional conversion in Turritopsis nutricula (Hydrozoa)".
- ↑ 69.0 69.1 69.2 Ruby, J. G., Smith, M., & Buffenstein, R. (2018). Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. elife, 7, e31157.
- ↑ Buffenstein, R., Amoroso, V., Andziak, B., Avdieiev, S., Azpurua, J., Barker, A. J., ... & Smith, E. S. J. (2021). The naked truth: a comprehensive clarification and classification of current ‘myths’ in naked mole‐rat biology. Biological Reviews.
- ↑ Kim, E. B., Fang, X., Fushan, A. A., Huang, Z., Lobanov, A. V., Han, L., ... & Gladyshev, V. N. (2011). Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature, 479(7372), 223-227.
- ↑ TEDx Talks. (2020, June 9). Ageing is a treatable disease | Nir Bazilai | TEDxBeaconStreetSalon [Video]. YouTube. https://www.youtube.com/watch?v=XN7rLbCBO1c
- ↑ https://icd.who.int/dev11/l-m/en?fbclid=IwAR22C-Gx2i9mckSYLenAwLAkWuBt3s3ncQLdjs5aar1W42jAbibuD6SQ2gE#/http://id.who.int/icd/entity/835503193
- ↑ https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f459275392
- ↑ 75.0 75.1 Scott, A. J., Ellison, M., & Sinclair, D. A. (2021). The economic value of targeting aging. Nature Aging, 1(7), 616-623.
- ↑ Who's Who in Gerontology: Researchers and Companies Working on Aging. (n.d.). Who's Who in Gerontology: Researchers and Companies Working on Aging. https://whoswho.senescence.info/
- ↑ Buck Institute. (n.d.). BUCK. https://www.buckinstitute.org/
- ↑ Welcome | The Sinclair Lab. (n.d.). Welcome | The Sinclair Lab. https://sinclair.hms.harvard.edu/
- ↑ Matt Kaeberlein, PhD | Faculty | Dept. of Laboratory Medicine & Pathology | UW Medicine. (n.d.). Dept. of Laboratory Medicine & Pathology | UW Medicine. https://dlmp.uw.edu/faculty/kaeberlein
- ↑ Brian Kennedy - Department of Biochemistry – School of Medicine, National University of Singapore. (n.d.). Department of Biochemistry – School of Medicine, National University of Singapore. https://medicine.nus.edu.sg/bch/fa
- ↑ Associate Prof Lynne Cox. (n.d.). Home | Biochemistry. https://www.bioch.ox.ac.uk/research/cox
- ↑ Endo A. (2010). A historical perspective on the discovery of statins. Proceedings of the Japan Academy. Series B, Physical and biological sciences, 86(5), 484–493. https://doi.org/10.2183/pjab.86.484
- ↑ Gavrilov, L. A., & Gavrilova, N. S. (2010). Demographic consequences of defeating aging. Rejuvenation research, 13(2-3), 329-334.
- ↑ The Cost of Sequencing a Human Genome. (n.d.). Genome.gov. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost
- ↑ Academy for Health & Lifespan Research. (n.d.). Academy for Health & Lifespan Research. https://www.ahlresearch.org/