NAD+

Nicotinamide adenine dinucleotide (NAD+) is a coenzyme found in all living cells. NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other a nicotinamide. It serves both as a critical coenzyme for enzymes that fuel reduction-oxidation reactions, carrying electrons from one reaction to another, and as a cosubstrate for other enzymes such as the sirtuins, CD38 and poly(adenosine diphosphate-ribose) polymerases (PARP).
Cellular NAD+ concentrations change during aging, and modulation of NAD+ usage or production has been proposed to prolong both healthspan and lifespan in animal models.[1][2][3][4][5]
The latest study from the ITP (Interventions Testing Program), which tests for the reproducibility of the lifespan effects from a range of compounds, showed that NAD+ supplementation had no effect in very old mice lifespan of either sex.[6] However, there is evidence that NAD+ might have beneficial effects in health in rather old mice.[2][4] For example, a potent and selective CD38 inhibitor, 78c, has been shown to restore low NAD+ levels in mouse models of aging, and thus protect against aging-induced health loss in aged male mice, resulting in an increase in lifespan (average by 17% and maximal by 14%).[7]
A bit of history
Links between NAD+ levels and health were established almost a century ago. In 1923-1936, Otto Warburg isolated, from an enzyme required for fermentation of sugar by yeast, two substrates: nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) and demonstrated theirs critical roles as indispensable cosubstrates in hydrogen transfer during fermentation.[8] In 1937, Conrad Elvehjem discovered that the disease pellagra (popularly known as Asturian leprosy or “mal de la rosa”, due to dermatitis with the reddish color of the skin rashes in areas of sun exposure, and also characterized by diarrhoea, dementia and other manifestations of premature aging)[9] resulted in low NAD+ and its phosphorylated counterpart NADP+ levels. He also found that this disease is caused by a dietary deficiency of the Pellagra preventing factors (PPF) which turned out to be niacin, also known as nicotinic acid or Vitamin B3.[10][11] Proteins rich in the amino acid tryptophan may also protect against pelagra, although the activity of tryptophan was 50 times lower than that of niacin.[12]
Later, low NAD+ levels were linked to multiple disease states, including metabolic and neurodegenerative diseases, and lower NAD+ levels are now known to correlate with ageing in rodents and humans. [4]
Importance in aging
NAD+ is an indispensable participant in the most important processes of energy metabolism, such as mitochondrial electron transport, glycolysis, and citric acid cycle.[13] Moreover, NAD+ is a rate-limiting substrate for many signalling enzymes such as sirtuin proteins SIRT1 and SIRT3, the poly (ADP-ribose) polymerase (PARP) proteins PARP1 and PARP2, a COOH-terminal binding proteins (CtBP transcriptional regulators), cyclic ADP-ribose (ADPR) synthetases CD38 and CD157, and many other NAD+ dependent enzymes.[13][14] These enzymes are involved in important cellular processes, such as DNA repair, stress response, genomic stability, chromatin remodelling, circadian rhythm regulation, cell cycle progression, insulin secretion and sensitivity, and expression of the inflammatory cytokines, thus translating changes in energy status into metabolic adaptations.[15]
Given their critical role as mediators of cellular responses to metabolic perturbations, it is unsurprising that dysregulation of NAD and NADP metabolism has been associated with the pathobiology of many chronic human diseases and aging.[4][16][17] NAD+ levels decrease with age due to increased DNA damage, oxidative stress, and chronic inflammation, which dysregulate NAD metabolism by activating CD38 and PARPs or by inhibiting NAMPT.[18][19][20]
NAD+ and NADP+

NAD+ and NADP+ are vital cofactors for most cellular oxidation/reduction reactions. Cellular NAD exists in two forms, oxidized (NAD+) and reduced (NADH). The NAD+/NADH couple primarily drives oxidation reactions, while the NADP+/NADPH couple drives reductive reactions.[21]
The mitochondrial tricarboxylic acid cycle (TCA) is a major location for the reduction of NAD+ into NADH molecules. Mitochondrial NADH can be re-oxidized to NAD+ by Complex I of the mitochondrial electron transport chain. The subsequent two electrons gained by Complex I will then be an initial step to generate a proton gradient that provides the chemiosmotic force to drive the oxidative phosphorylation of ADP to ATP. These processes highlight the intimate link between NAD+ and cellular ATP synthesis.
By accepting and donating hydride ions (H−), NAD+, as an oxidoreductase cofactor, plays a central role in metabolism, supporting myriad biochemical reactions including of glycolysis, oxidative phosphorylation, and β-oxidation. At the same time, ATP generated via glycolytic reactions is critical for NAD+ regeneration from NADH by mitochondrial complex I of the electron transport chain (ETC). Through this process, mitochondria can communicate with the rest of the cell and thereby regulate physiological and pathophysiological outcomes.[22] Senescent cells have been shown to be less efficient in producing ATP due to the decrease in the efficiency of oxidative phosphorylation (OXPHOS), characterized by less H+ in the intermembrane space, i.e. with a reduction of mitochondrial membrane potential. While decrease in mitochondrial membrane potential was associated with increased production of ROS (reactive oxygen species), suggesting that mitochondria in senescent cells are dysfunctional.[23] Many types of senescent cells show increased mitochondrial mass per cell. But this does not compensate enough for the decrease in the functionality of aged mitochondria.[24] However, treatment with KB1541 that enhances the efficiency of OXPHOS is accompanied by amelioration of senescent phenotypes, rendering targeting mitochondrial metabolic reprogramming as a potential treatment method for senescence.[25]
Sirtuins
The remarkably conserved enzymes throughout evolution from archaebacteria to eukaryotes named sirtuins have been defined as a family of nicotinamide adenine dinucleotide-dependent enzymes that deacetylate lysine residue on various proteins. Certain sirtuins have in addition an ADP-ribosyltransferase activity. Their exceptional conservation indicates that these proteins play vital physiological roles.[26] Sirtuins are the major players in delaying biological aging. Sirtuins depend on NAD+ for their activity. Therefore, the availability of NAD+ is a boost for the action of sirtuins.[27][28] However, how NAD deficiency may affect longevity through the sirtuins inhibition is not entirely clear. On the one hand brain-specific Sirt1-overexpressing (BRASTO) transgenic mice show significant life span extension in both males and females and phenotypes consistent with a delay in aging,[29] Inhancing the function of SIRT6 also improves the healthspan in mice,[30][31] while on the other hand, male, but not female, Sirt7 knockout mice exhibited an extension of mean and maximum lifespan, displayed better glucose tolerance with improved insulin sensitivity and a delay in the age-associated mortality rate compared with wild-type mice.[32] Such opposite roles in aging of SIRT1/SIRT6 and SIRT7 may actually be associated with different dynamics of the amount of SIRT7 in different organs. Since, significant reductions in SIRT7 levels have been reported in most organs and tissues of human and animal models as a consequence of aging, including the heart, liver, lungs, colon, skin, subcutaneous white adipose tissue (WAT) depots, hair follicles, and blood, while, there are some tissues in which SIRT7 levels increase with aging, such as in the frontal lobe of the brain and retroperitoneal WAT depots.[33]
CtBP family of NADH-dependent transcriptional regulators
CtBP (C-terminal binding protein) is an evolutionarily conserved NAD(H)-dependent transcriptional corepressor, whose activity has been shown to be regulated by the NAD/NADH ratio.[34] Increasing cellular NADH/NAD+ ratio, like under hypoxia condition, promotes CtBP1 repression activity leading to transcriptional repression of the target genes.[35] Loss of CtBP either by depletion or mutation triggered an extended life span in C. elegans.[36][37]
Mammalian genomes encode two CtBPs, CtBP1 and CtBP2, which function as both corepressors and coactivators in different biological processes ranging from apoptosis to inflammation and osteogenesis. Their overexpression in tumors is associated with malignant behavior, such as uncontrolled cell proliferation, migration, and invasion, as well as with an increase in the epithelial-mesenchymal transition.[38]
Since CtBP1 and CtBP2 do not bind directly to DNA, they regulate cellular processes through binding transcription factors and recruiting chromatin remodeling enzymes such as histone deacetylases, methyl transferases, and demethylases to targeted promoters by developing dimers with DNA-binding proteins.[39][40]
Studies in model organisms have demonstrated that CtBP is indispensable for embryonic development and adult lifespan regulation. The homozygous mutation of mCtBP2 in mouse leads to developmental defects and embryonic death, while mCtBP1 homozygous deletion reduces their offsprings’ life span.[41]
CtBP plays a prominent role in repression of E-cadherin, which suppresses tumorigenesis by restricting tumor cell motility and invasion.[42] Protocatechuic aldehyde, a natural compound in the root of a traditional Chinese herb, Salvia miltiorrhiza, that inhibited the proliferation and migration of breast cancer cells, could be a potential CtBP1 inhibitor, due to its ability to directly attach to the CtBP1 and specifically attenuate the repression activity of CtBP1 on p21 and E-cadherin.[42]
NAMPT

The protein NAMPT, encoded by the NAPMT gene, is present in intracellular form of NAMPT (iNAMPT) and as secreted by cells and referred to as extracellular NAMPT (eNAMPT also known as Visfatin and as PBEF (pre-B-cell colony-enhancing factor), and was initially described as an adipokine.[44]
NAMPT/Visfatin/PBEF (nicotinamide phosphoribosyltransferase) catalyses the transfer of a phosphoribosyl group from PRPP (5-phosphoribosyl-1-pyrophosphate) to nicotinamide, forming NMN (nicotinamide mononucleotide) and PPi (pyrophosphate). NMN is then converted to NAD by Nmnat (nicotinamide mononucleotide adenylyltransferase).[43][45]
NAMPT is the main bottleneck in NAD+ biosynthetic pathway making it a regulator of the intracellular NAD pool. Thus, NAMPT influences the activity of NAD-dependent enzymes, thereby regulating cellular metabolism.[45]
NAD-Capped RNAs
Metabolites like NAD were found to function as 5′-cap structures of RNA. It is assumed that NAD-RNA defines a fundamental regulatory mechanism at the epitranscriptomic level.[46] Interestingly, despite the fact that NAD decreases with age, it was found that the number of NAD-capping events tended to increase in aged human subject.[47] A set of NAD-RNAs that are highly associated with age have been identified.[47] Specifically, select NAD-RNAs, such as those involved in protein folding (PDIA3), protein ubiquitination (SUMO1), and apoptosis (caspase 3 and 8), had increased capping with age, although the abundance at RNA transcript levels was not increased.[47] At the same time, NAD-capping genes linked to mRNA decay (UPF2), calmodulin binding (NRGN), and TGF-β signaling pathway (TGFB1) were decreased during aging.[47]
Ways of boosting NAD+ in aging
Although NAD+/NADH in most people declines in aged tissues, specifically in the brain,[48] skeletal muscles[49][50] and plasma,[51] no reduced NAD+/NADH levels were found in centenarians in the plasma when compared to young individuals.[52] Many studies and clinical trials are currently being carried out increasing the levels of NAD+ in the organisms, both using precursors to increase its levels by intake (NR, NMN and Vitamin B3), or inhibiting NAD+ consumption by inhibiting NAD+ consuming enzymes (e.g. PARP and CD38) to limit its depletion.[53][54][55][56][57]
Counteracting NAD+ deficiency with NAD+ precursors
Boosting intracellular NAD+ content has been suggested as a potential anti-aging strategy.[58][59][60][61] Despite limited conclusive evidence, supplements of NAD+ precursors, namely nicotinamide (NAM), nicotinic acid (NA)[62][63], nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), aimed at increasing NAD+ levels are becoming increasingly popular.[64] In addition, nutritional supplementation of trigonelline could serve as a NAD+ boosting strategy. [65]
While it is anticipated that NAD+ precursors can play beneficial protective roles in several conditions, they vary in their ability to promote NAD+ anabolism with differing adverse effects. Careful evaluation of the role of NAD+, whether friend or foe in ageing, should be considered.[18]
GPR109A
GPR109A receptor also known as hydroxycarboxylic acid receptor 2 (HCAR2), niacin receptor 1 (NIACR1), HM74a, HM74b, and PUMA-G is located on chromosome 12 (Band 12q24.31) in humans.[66] The most notable agonist for GPR109A is niacin.[67] The other endogenous agonists of GPR109A are Beta hydroxy butyrate (BHB) and butyrate, which are ketone bodies produced during ketosis. GPR109A is involved in the vascular inflammation pathway related to the antiatherosclerotic effect of niacin.[68] GPR109A have long represented the molecular target for the anti-dyslipidemic actions of niacin and the endogenous ligand 3-hydroxy-butyric acid, being enriched on adipocytes.[69] Niacin (nicotinic acid) at high doses favorably modulates the human lipid profile by elevating high-density lipoprotein cholesterol (HDL-C) and decreasing low-density lipoprotein cholesterol (LDL-C) and lipoprotein a [Lp(a)][70][71][72] associated with a reduced risk of mortality.[73][74] Nicotinamide riboside (NR) administration is a valid tool to boost NAD+ levels in mammalian cells and tissues but without activating GPR109A and so without antiatherosclerotic effect.[75] It is interesting to note that besides niacin, some other small molecules are able to activate the GPR109A receptor, for example non-flushing[76] MK-6892,[77][78], not successful GSK256073,[79] and recently approved monomethyl fumarate (MMF, Bafiertam).[80]
NAD+ regulation by microbiome

NAD precursors obtained from the diet are complemented by NA synthesized by a healthy gut microbiome. The gut microbiota uses host-derived nicotinamide to generate NAD and in return, produces nicotinic acid for host NAD biosynthesis. Furthermore, the main route from oral nicotinamide riboside, a widely used nutraceutical, to host NAD is via conversion into nicotinic acid by the gut microbiome.[81][82][83]
PARPs
Poly(ADP-ribose) polymerases (PARPs), also known as ADP-ribosyltransferases (ARTs), are a family of proteins that catalyzes either mono-ADP-ribose (MAR) or poly ADP-ribose (PAR) to target proteins using NAD+ as a donor; this process is also termed MARylation or PARylation, respectively.[84] PARP is an abundant ADP-ribosyltransferase that functions as a DNA nick-sensor and contributes to DNA repair, chromatin remodeling, and genomic stability. In particular, PARP1 and PARP2 play a role in the base excision repair (BER) pathway, while PARP3 senses double-strand breaks (DSBs) and is involved in double-strand break repair (DSBR).
In response to age-dependent accumulation of DNA damage, PARPs consume more NAD+ resulting in reduced cellular/tissue NAD+. In particular, PARP1 uses NAD+ to generate large amounts of poly(ADP-ribose), which promotes the recruitment of DNA repair factors. However, excessive activation of PARP1 causes depletion of intracellular NAD+ and ATP levels, eventually leading to cell death.[84] PARP1 activation allows the recruitment of DNA repair proteins to repair damaged DNA. Although PARP1 activation is crucial for genomic maintenance, hyperactivation of PARP1 can cause a reduction in NAD+ levels.[85][86]
CD38 and CD157
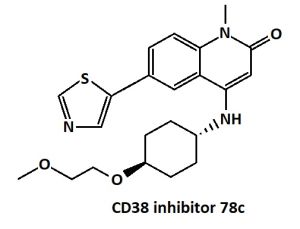
CD38 is an enzyme critical for the regulation of NAD+ levels. Senescent cells promote tissue NAD+ decline during ageing mainly via the activation of CD38+ macrophages.[87]
CD38 and its sister molecule CD157[88] belong to a family with ADP-ribosyl cyclase activity involved in the regulation of calcium mobilization processes from ryanodine receptors to intracellular Ca2+ pools by cyclic ADP-ribose (cADPR).[89] They are involved in the formation of cADPR from NAD+. The ADP-ribosyl cyclase activity of CD157 is weaker than that of CD38.[90] The function of CD157 is mediated solely by the second messenger cyclic ADP-ribose and not nicotinic acid adenine dinucleotide phosphate (NAADP).[91] While CD38 is known to have a robust ability to catalyze cADPR as well as NAADP formation, that plays an important role in insulin secretion.[92]
Many studies have reported that a flavonoid compound cyanidin-3-O-glucoside (C3G), a natural inhibitor of CD38, that prevent CD38 from consuming NAD+[93] exerts anti-tumor, anti-inflammatory and antioxidant effects.[94][95][96]
References
- ↑ Lautrup, S.Hou, Y.Fang, E. F.Bohr, V. A. (2023). Roles of NAD+ in Health and Aging. Cold Spring Harb Perspect Med 2024; 14: a041320, doi: 10.1101/cshperspect.a041193 doi: 10.1101/cshperspect.a041165
- ↑ 2.0 2.1 Poljšak, B., Kovač, V., Špalj, S., & Milisav, I. (2023). The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. International Journal of Molecular Sciences, 24(3), 2959. https://doi.org/10.3390/ijms24032959
- ↑ Verdin, E. (2015). NAD⁺ in aging, metabolism, and neurodegeneration. Science (New York, NY), 350(6265), 1208-1213. PMID: 26785480 DOI: 10.1126/science.aac4854
- ↑ 4.0 4.1 4.2 4.3 Covarrubias, A. J., Perrone, R., Grozio, A., & Verdin, E. (2021). NAD+ metabolism and its roles in cellular processes during ageing. Nature Reviews Molecular Cell Biology, 22(2), 119-141. PMID: 33353981 PMC7963035 DOI: 10.1038/s41580-020-00313-x
- ↑ Chini, C. C. S., Cordeiro, H. S., Tran, N. L. K., & Chini, E. N. (2023). NAD metabolism: Role in senescence regulation and aging. Aging Cell, e13920. PMID: 37424179 https://doi.org/10.1111/acel.13920
- ↑ Harrison, D. E., Strong, R., Reifsnyder, P., Kumar, N., Fernandez, E., Flurkey, K., ... & Miller, R. A. (2021). 17‐a‐estradiol late in life extends lifespan in aging UM‐HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell, 20(5), e13328.
- ↑ 7.0 7.1 Peclat, T. R., Thompson, K. L., Warner, G. M., Chini, C. C., Tarragó, M. G., Mazdeh, D. Z., ... & Chini, E. N. (2022). CD38 inhibitor 78c increases mice lifespan and healthspan in a model of chronological aging. Aging Cell, e13589. PMID:35263032 doi:10.1111/acel.13589
- ↑ Fessel, J. P., & Oldham, W. M. (2018). Pyridine dinucleotides from molecules to man. Antioxidants & redox signaling, 28(3), 180-212. PMID: 28635300 PMC[1] DOI: 10.1089/ars.2017.7120
- ↑ Johnson L.E. (2022). Niacin Deficiency (Pellagra). MSD Manual. Merck & Co., Inc. https://www.msdmanuals.com/home/disorders-of-nutrition/vitamins/niacin-deficiency
- ↑ Rachmilewitz, M., & Glueck, H. I. (1938). Treatment of pellagra with nicotinic acid. British Medical Journal, 2(4049), 346. PMID:20781664 PMC2210222 DOI: 10.1136/bmj.2.4049.346
- ↑ Williams, A. C., & Ramsden, D. B. (2007). Pellagra: A clue as to why energy failure causes diseases?. Medical hypotheses, 69(3), 618-628. PMID: 17349750 DOI: 10.1016/j.mehy.2007.01.029
- ↑ Krehl, W. A., Teply, L. J., Sarma, P. S., & Elvehjem, C. A. (1945). Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophane. Science, 101(2628), 489-490. PMID: 17735529 DOI: 10.1126/science.101.2628.489
- ↑ 13.0 13.1 Xie, N., Zhang, L., Gao, W., Huang, C., Huber, P. E., Zhou, X., ... & Zou, B. (2020). NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal transduction and targeted therapy, 5(1), 1-37. PMID: 33028824 PMC7539288 DOI: 10.1038/s41392-020-00311-7
- ↑ Poljsak, B., Kovač, V., & Milisav, I. (2020). Healthy lifestyle recommendations: Do the beneficial effects originate from NAD+ amount at the cellular level?. Oxidative medicine and cellular longevity, 2020. 8819627. PMID: 33414897 PMC7752291 DOI: 10.1155/2020/8819627
- ↑ Amjad, S., Nisar, S., Bhat, A. A., Frenneaux, M. P., Fakhro, K., Haris, M., ... & Bagga, P. (2021). Role of NAD+ in regulating cellular and metabolic signaling pathways. Molecular Metabolism, 49, 101195. PMID: 33609766 PMC7973386 DOI: 10.1016/j.molmet.2021.101195
- ↑ Braidy, N., Berg, J., Clement, J., Khorshidi, F., Poljak, A., Jayasena, T., ... & Sachdev, P. (2019). Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxidants & Redox Signaling, 30(2), 251-294. PMID: 29634344 PMC6277084 DOI: 10.1089/ars.2017.7269
- ↑ Yaku, K., Okabe, K., & Nakagawa, T. (2018). NAD metabolism: Implications in aging and longevity. Ageing research reviews, 47, 1-17. PMID: 29883761 DOI: 10.1016/j.arr.2018.05.006
- ↑ 18.0 18.1 Poljšak, B., Kovač, V., & Milisav, I. (2022). Current Uncertainties and Future Challenges Regarding NAD+ Boosting Strategies. Antioxidants, 11(9), 1637. PMID: 36139711 PMC9495723 DOI: 10.3390/antiox11091637
- ↑ McReynolds, M. R., Chellappa, K., Chiles, E., Jankowski, C., Shen, Y., Chen, L., ... & Baur, J. A. (2021). NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Systems, 12(12), 1160-1172. PMID: 34559996 PMC8678178 DOI: 10.1016/j.cels.2021.09.001
- ↑ Cercillieux, A., Ciarlo, E., & Canto, C. (2022). Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations. Cellular and Molecular Life Sciences, 79(8), 1-28. PMID:35918544 PMC9345839 DOI: 10.1007/s00018-022-04499-5
- ↑ Xiao, W., Wang, R. S., Handy, D. E., & Loscalzo, J. (2018). NAD (H) and NADP (H) redox couples and cellular energy metabolism. Antioxidants & redox signaling, 28(3), 251-272. PMID: 28648096 PMC5737637 DOI: 10.1089/ars.2017.7216
- ↑ Chakrabarty, R. P., & Chandel, N. S. (2022). Beyond ATP, new roles of mitochondria. The Biochemist, 44(4), 2-8. PMID:36248614 PMC9558425 DOI:10.1042/bio_2022_119
- ↑ Stöckl, P., Hütter, E., Zwerschke, W., & Jansen-Dürr, P. (2006). Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Experimental gerontology, 41(7), 674-682. PMID: 16713693 DOI: 10.1016/j.exger.2006.04.009
- ↑ Martini, H., & Passos, J. F. (2022). Cellular senescence: all roads lead to mitochondria. The FEBS Journal. 107, 216-227 PMID: 35048548 PMC9296701 DOI:10.1111/febs.16361
- ↑ Lee, Y. H., Choi, D., Jang, G., Park, J. Y., Song, E. S., Lee, H., ... & Park, J. T. (2022). Targeting regulation of ATP synthase 5 alpha/beta dimerization alleviates senescence. Aging (Albany NY), 14(2), 678. PMID: 35093936 PMC8833107 DOI: 10.18632/aging.203858
- ↑ Gaur, U., Tu, J., Li, D., Gao, Y., Lian, T., Sun, B., ... & Yang, M. (2017). Molecular evolutionary patterns of NAD+/Sirtuin aging signaling pathway across taxa. PloS one, 12(8), e0182306. PMID: 28767699 PMC5540417 DOI: 10.1371/journal.pone.0182306
- ↑ He, E. (2021). The relationship between NAD+ and Sirtuins in aging.
- ↑ Wang, M., & Lin, H. (2021). Understanding the function of mammalian sirtuins and protein lysine acylation. Annual Review of Biochemistry, 90, 245-285. PMID: 33848425 DOI: 10.1146/annurev-biochem-082520-125411
- ↑ Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., ... & Imai, S. I. (2013). Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell metabolism, 18(3), 416-430.
- ↑ Roichman, A., Elhanati, S., Aon, M. A., Abramovich, I., Di Francesco, A., Shahar, Y., ... & Cohen, H. Y. (2021). Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nature communications, 12(1), 1-18. PMID: 34050173 PMC8163764 DOI: 10.1038/s41467-021-23545-7
- ↑ Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., ... & Cohen, H. Y. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature, 483(7388), 218-221. PMID: 22367546 DOI: 10.1038/nature10815
- ↑ Mizumoto, T., Yoshizawa, T., Sato, Y., Ito, T., Tsuyama, T., Satoh, A., ... & Yamagata, K. (2022). SIRT7 Deficiency Protects against Aging-Associated Glucose Intolerance and Extends Lifespan in Male Mice. Cells, 11(22), 3609. https://doi.org/10.3390/cells11223609
- ↑ Lagunas-Rangel, F. A. (2022). SIRT7 in the aging process. Cellular and Molecular Life Sciences, 79(6), 1-17 PMID: 35585284 PMC9117384 DOI: 10.1007/s00018-022-04342-x.
- ↑ Chinnadurai, G. (2007). Transcriptional regulation by C-terminal binding proteins. The international journal of biochemistry & cell biology, 39(9), 1593-1607. PMID: 17336131 DOI: 10.1016/j.biocel.2007.01.025
- ↑ Deng, Y., Liu, J., Han, G., Lu, S. L., Wang, S. Y., Malkoski, S., ... & Zhang, Q. (2010). Redox-dependent Brca1 transcriptional regulation by an NADH-sensor CtBP1. Oncogene, 29(50), 6603-6608. PMID: 20818429 PMC3081720 DOI: 10.1038/onc.2010.406
- ↑ Reid, A., Yücel, D., Wood, M., Llamosas, E., Kant, S., Crossley, M., & Nicholas, H. (2014). The transcriptional repressor CTBP-1 functions in the nervous system of Caenorhabditis elegans to regulate lifespan. Experimental gerontology, 60, 153-165. PMID: 25456848 DOI: 10.1016/j.exger.2014.09.022
- ↑ Chen, S., Whetstine, J. R., Ghosh, S., Hanover, J. A., Gali, R. R., Grosu, P., & Shi, Y. (2009). The conserved NAD (H)-dependent corepressor CTBP-1 regulates Caenorhabditis elegans life span. Proceedings of the National Academy of Sciences, 106(5), 1496-1501. PMID: 19164523 PMC2635826 DOI: 10.1073/pnas.0802674106
- ↑ Dcona, M. M., Morris, B. L., Ellis, K. C., & Grossman, S. R. (2017). CtBP-an emerging oncogene and novel small molecule drug target: Advances in the understanding of its oncogenic action and identification of therapeutic inhibitors. Cancer Biology & Therapy, 18(6), 379-391. PMID: 28532298 PMC5536941 DOI: 10.1080/15384047.2017.1323586
- ↑ Stankiewicz, T. R., Gray, J. J., Winter, A. N., & Linseman, D. A. (2014). C-terminal binding proteins: central players in development and disease. Biomolecular concepts, 5(6), 489-511. PMID: 25429601 DOI: 10.1515/bmc-2014-0027
- ↑ Derleth, M. R., Avila, A., Atchison, M., & Basu, A. (2020). Interplay of YY1 and CtBP in the recruitment of PcG complexes to DNA. The FASEB Journal, 34(S1), 1-1. https://doi.org/10.1096/fasebj.2020.34.s1.03988
- ↑ Hildebrand, J. D., & Soriano, P. (2002). Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Molecular and cellular biology, 22(15), 5296-5307. PMID: 12101226 PMC133942 DOI: 10.1128/MCB.22.15.5296-5307.2002
- ↑ 42.0 42.1 Deng, Y., Guo, W., Li, G., Li, S., Li, H., Li, X., ... & Li, F. (2020). Protocatechuic Aldehyde Represses Proliferation and Migration of Breast Cancer Cells through Targeting C-terminal Binding Protein 1. Journal of Breast Cancer, 23(1), 20-35. PMID: 32140267 PMC7043946 DOI: 10.4048/jbc.2020.23.e7
- ↑ 43.0 43.1 Garten, A., Petzold, S., Körner, A., Imai, S. I., & Kiess, W. (2009). Nampt: linking NAD biology, metabolism and cancer. Trends in Endocrinology & Metabolism, 20(3), 130-138. PMID: 19109034 PMC2738422 DOI: 10.1016/j.tem.2008.10.004
- ↑ Revollo, J. R., Grimm, A. A., & Imai, S. I. (2007). The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Current opinion in gastroenterology, 23(2), 164-170. PMID: 17268245 DOI: 10.1097/MOG.0b013e32801b3c8f
- ↑ 45.0 45.1 Zhu, Y., Xu, P., Huang, X., Shuai, W., Liu, L., Zhang, S., ... & Wang, G. (2022). From Rate-Limiting Enzyme to Therapeutic Target: The Promise of NAMPT in Neurodegenerative Diseases. Frontiers in Pharmacology, 13. PMID: 35903330 PMC9322656 DOI: 10.3389/fphar.2022.920113
- ↑ Ge, S., Wang, X., Wang, Y., Dong, M., Li, D., Niu, K., ... & Zhong, M. (2024). Hidden features of NAD-RNA epitranscriptome in Drosophila life cycle. Iscience, 27(1).
- ↑ 47.0 47.1 47.2 47.3 Li, D., & Liu, N. (2023). Epitranscriptome analysis of NAD-capped RNA by spike-in-based normalization. bioRxiv, 2023-03. https://doi.org/10.1101/2023.03.23.534034
- ↑ Zhu, X. H., Lu, M., Lee, B. Y., Ugurbil, K., & Chen, W. (2015). In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proceedings of the National Academy of Sciences, 112(9), 2876-2881. PMID: 25730862 PMC4352772 DOI: 10.1073/pnas.1417921112
- ↑ Mendelsohn, A. R., & Larrick, J. W. (2014). Partial reversal of skeletal muscle aging by restoration of normal NAD+ levels. Rejuvenation research, 17(1), 62-69. PMID: 24410488 DOI: 10.1089/rej.2014.1546
- ↑ Wagner, S., Manickam, R., Brotto, M., & Tipparaju, S. M. (2022). NAD+ centric mechanisms and molecular determinants of skeletal muscle disease and aging. Molecular and Cellular Biochemistry, 1-20. PMID: 35334034 DOI: 10.1007/s11010-022-04408-1
- ↑ Clement, J., Wong, M., Poljak, A., Sachdev, P., & Braidy, N. (2019). The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation research, 22(2), 121-130. PMID: 30124109 PMC6482912 DOI: 10.1089/rej.2018.2077
- ↑ Sanchez-Roman, I., Ferrando, B., Holst, C. M., Mengel-From, J., Rasmussen, S. H., Thinggaard, M., ... & Stevnsner, T. (2022). Molecular markers of DNA repair and brain metabolism correlate with cognition in centenarians. GeroScience, 44(1), 103-125. PMID: 34966960 PMC8810979 DOI: 10.1007/s11357-021-00502-2
- ↑ Chu, X., & Raju, R. P. (2022). Regulation of NAD+ metabolism in aging and disease. Metabolism, 126, 154923. PMID: 34743990 PMC8649045 DOI: 10.1016/j.metabol.2021.154923
- ↑ Conlon, N., & Ford, D. (2022). A systems-approach to NAD+ restoration. Biochemical pharmacology, 114946. PMID: 35134387 DOI:10.1016/j.bcp.2022.114946
- ↑ Helman, T., & Braidy, N. (2022). Importance of NAD+ Anabolism in Metabolic, Cardiovascular and Neurodegenerative Disorders. Drugs & Aging, 1-16. PMID: 36510042 DOI: 10.1007/s40266-022-00989-0
- ↑ Reiten, O. K., Wilvang, M. A., Mitchell, S. J., Hu, Z., & Fang, E. F. (2021). Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mechanisms of Ageing and Development, 199, 111567. PMID: 34517020 DOI: 10.1016/j.mad.2021.111567
- ↑ Sharma, A., Chabloz, S., Lapides, R. A., Roider, E., & Ewald, C. Y. (2023). Potential Synergistic Supplementation of NAD+ Promoting Compounds as a Strategy for Increasing Healthspan. Nutrients, 15(2), 445. DOI:10.3390/nu15020445
- ↑ Yang, T., Chan, N. Y. K., & Sauve, A. A. (2007). Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. Journal of medicinal chemistry, 50(26), 6458-6461. PMID: 18052316 DOI: 10.1021/jm701001c
- ↑ Bonkowski, M. S., & Sinclair, D. A. (2016). Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nature reviews Molecular cell biology, 17(11), 679-690. PMID: 27552971 PMC5107309 DOI: 10.1038/nrm.2016.93
- ↑ Wu, L. E., & Sinclair, D. A. (2016). Restoring stem cells—all you need is NAD+. Cell Research, 26(9), 971-972. PMID: 27339086 PMC5034109 DOI: 10.1038/cr.2016.80
- ↑ Sharma, C., Donu, D., & Cen, Y. (2022). Emerging Role of Nicotinamide Riboside in Health and Diseases. Nutrients, 14(19), 3889. Nutrients 2022, 14(19), 3889; PMID: 36235542 PMC9571518 DOI:10.3390/nu14193889
- ↑ Pirinen, E., Auranen, M., Khan, N. A., Brilhante, V., Urho, N., Pessia, A., ... & Suomalainen, A. (2020). Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell metabolism, 31(6), 1078-1090. PMID: 32386566 DOI: 10.1016/j.cmet.2020.04.008
- ↑ Niacin Increases NAD (Test Results)
- ↑ Palmer, R. D., Elnashar, M. M., & Vaccarezza, M. (2021). Precursor comparisons for the upregulation of nicotinamide adenine dinucleotide. Novel approaches for better aging. Aging Medicine, 4(3), 214-220. PMID: 34553119 PMC8444956 DOI: 10.1002/agm2.12170
- ↑ Membrez, M., Migliavacca, E., Christen, S., Yaku, K., Trieu, J., Lee, A. K., ... & Feige, J. N. (2024). Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nature Metabolism, 6, 433–447 https://doi.org/10.1038/s42255-024-00997-x
- ↑ Zellner, C., Pullinger, C. R., Aouizerat, B. E., Frost, P. H., Kwok, P. Y., Malloy, M. J., & Kane, J. P. (2005). Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors. Human mutation, 25(1), 18-21. PMID: 15580557 DOI: 10.1002/humu.20121
- ↑ Taing, K., Chen, L., & Weng, H. R. (2023). Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain. Neural Regeneration Research, 18(4), 763. PMID: 36204834 PMC9700108 DOI: 10.4103/1673-5374.354514
- ↑ Lukasova, M., Malaval, C., Gille, A., Kero, 1. J., & Offermanns, S. (2011). Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. The Journal of clinical investigation, 121(3), 1163-1173. http://www.jci.org/articles/view/41651.
- ↑ Yang, Y., Kang, H. J., Gao, R., Wang, J., Han, G. W., DiBerto, J. F., ... & Liu, Z. J. (2023). Structural insights into the human niacin receptor HCA2-Gi signalling complex. Nature Communications, 14(1), 1692. PMID: 36973264 PMCID: PMC10043007 DOI: 10.1038/s41467-023-37177-6
- ↑ Altschul, R., Hoffer, A., & Stephen, J. D. (1955). Influence of nicotinic acid on serum cholesterol in man. Archives of Biochemistry, 54, 558-559.
- ↑ Lampsas, S., Xenou, M., Oikonomou, E., Pantelidis, P., Lysandrou, A., Sarantos, S., ... & Siasos, G. (2023). Lipoprotein (a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules, 28(3), 969. PMID: 36770634 PMC9918959 DOI: 10.3390/molecules28030969
- ↑ Kühnast, S., Louwe, M. C., Heemskerk, M. M., Pieterman, E. J., van Klinken, J. B., van den Berg, S. A., ... & Jukema, J. W. (2013). Niacin reduces atherosclerosis development in APOE* 3Leiden. CETP mice mainly by reducing nonHDL-cholesterol. PloS one, 8(6), e66467. PMID: 23840481 PMCID: PMC3686722 DOI: 10.1371/journal.pone.0066467
- ↑ Canner, P. L., Berge, K. G., Wenger, N. K., Stamler, J., Friedman, L., Prineas, R. J., ... & Coronary Drug Project Research Group. (1986). Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. Journal of the American College of Cardiology, 8(6), 1245-1255.
- ↑ Tang, C., Eshak, E. S., Shirai, K., Tamakoshi, A., & Iso, H. (2023). Associations of dietary intakes of vitamins B1 and B3 with risk of mortality from CVD among Japanese men and women: the Japan Collaborative Cohort study. British Journal of Nutrition, 129(7), 1213-1220. PMID: 35466893 PMCID: PMC10011590 DOI: 10.1017/S0007114522001209
- ↑ Cantó, C., Houtkooper, R. H., Pirinen, E., Youn, D. Y., Oosterveer, M. H., Cen, Y., ... & Auwerx, J. (2012). The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism, 15(6), 838-847. PMID: 22682224 PMC3616313 DOI: 10.1016/j.cmet.2012.04.022
- ↑ Walters, R. W., Shukla, A. K., Kovacs, J. J., Violin, J. D., DeWire, S. M., Lam, C. M., ... & Lefkowitz, R. J. (2009). β-Arrestin1 mediates nicotinic acid–induced flushing, but not its antilipolytic effect, in mice. The Journal of clinical investigation, 119(5), 1312-1321. PMID: 19349687 PMC2673863 DOI: 10.1172/JCI36806
- ↑ Shen, H. C., Ding, F. X., Raghavan, S., Deng, Q., Luell, S., Forrest, M. J., ... & Colletti, S. L. (2010). Discovery of a biaryl cyclohexene carboxylic acid (MK-6892): a potent and selective high affinity niacin receptor full agonist with reduced flushing profiles in animals as a preclinical candidate. Journal of medicinal chemistry, 53(6), 2666-2670. PMID: 20184326 DOI: 10.1021/jm100022r
- ↑ Kim, H. Y., Jadhav, V. B., Jeong, D. Y., Park, W. K., Song, J. H., Lee, S., & Cho, H. (2015). Discovery of 4-(phenyl) thio-1 H-pyrazole derivatives as agonists of GPR109A, a high affinity niacin receptor. Archives of pharmacal research, 38, 1019-1032. PMID: 25599616 DOI: 10.1007/s12272-015-0560-4
- ↑ Olson, E. J., Mahar, K. M., Haws, T. F., Fossler, M. J., Gao, F., de Gouville, A. C., ... & Lepore, J. J. (2019). A Randomized, Placebo‐Controlled Trial to Assess the Effects of 8 Weeks of Administration of GSK256073, a Selective GPR109A Agonist, on High‐Density Lipoprotein Cholesterol in Subjects With Dyslipidemia. Clinical Pharmacology in Drug Development, 8(7), 871-883. PMID: 31268250 DOI: 10.1002/cpdd.704
- ↑ Yadav, M., Sarma, P., Ganguly, M., Mishra, S., Maharana, J., Zaidi, N., ... & Shukla, A. K. (2023). Structure-guided engineering of biased-agonism in the human niacin receptor via single amino acid substitution. bioRxiv, 2023-07. https://doi.org/10.1101/2023.07.03.547505
- ↑ Chellappa, K., McReynolds, M. R., Lu, W., Zeng, X., Makarov, M., Hayat, F., ... & Baur, J. A. (2022). NAD precursors cycle between host tissues and the gut microbiome. Cell Metabolism, 34(12), 1947-1959. PMID: 36476934 PMC9825113 DOI: 10.1016/j.cmet.2022.11.004
- ↑ Shats, I., Williams, J. G., Liu, J., Makarov, M. V., Wu, X., Lih, F. B., ... & Li, X. (2020). Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell metabolism, 31(3), 564-579. PMID: 32130883 PMC7194078 DOI: 10.1016/j.cmet.2020.02.001
- ↑ Liu, L., Su, X., Quinn, W. J., Hui, S., Krukenberg, K., Frederick, D. W., ... & Rabinowitz, J. D. (2018). Quantitative analysis of NAD synthesis-breakdown fluxes. Cell metabolism, 27(5), 1067-1080.PMID: 29685734 PMC5932087 DOI: 10.1016/j.cmet.2018.03.018
- ↑ 84.0 84.1 Gupte, R., Liu, Z., & Kraus, W. L. (2017). PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes & development, 31(2), 101-126. PMID:28202539 PMC5322727 DOI: 10.1101/gad.291518.116
- ↑ Maynard, S., Fang, E. F., Scheibye-Knudsen, M., Croteau, D. L., & Bohr, V. A. (2015). DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harbor perspectives in medicine, 5(10), a025130. PMID: 26385091 PMC4588127 DOI: 10.1101/cshperspect.a025130
- ↑ Birkisdóttir, M. B., van Galen, I., Brandt, R., Barnhoorn, S., van Vliet, N., van Dijk, C., ... & Vermeij, W. P. (2022). The use of progeroid DNA repair-deficient mice for assessing anti-aging compounds, illustrating the benefits of nicotinamide riboside. Frontiers in Aging, 3: 1005322. PMID: 36313181 PMC9596940 DOI:10.3389/fragi.2022.1005322
- ↑ Covarrubias, A. J., Kale, A., Perrone, R., Lopez-Dominguez, J. A., Pisco, A. O., Kasler, H. G., ... & Verdin, E. (2020). Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nature metabolism, 2(11), 1265-1283. PMID: 33199924 PMC7908681 DOI: 10.1038/s42255-020-00305-3
- ↑ Ishihara, K. (2014). History and perspectives of research in bone marrow stromal cell antigen-1 (BST-1)/CD157: a relative of ADP-ribosyl cyclase CD38. Messenger, 3(1-2), 15-20. https://doi.org/10.1166/msr.2014.1038
- ↑ Quarona, V., Zaccarello, G., Chillemi, A., Brunetti, E., Singh, V. K., Ferrero, E., ... & Malavasi, F. (2013). CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry Part B: Clinical Cytometry, 84(4), 207-217. PMID: 23576305 DOI:10.1002/cyto.b.21092
- ↑ Lee, H. C. (2012). Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. Journal of Biological Chemistry, 287(38), 31633-31640. PMID: 22822066 PMC3442497 DOI: 10.1074/jbc.R112.349464
- ↑ Higashida, H., Liang, M., Yoshihara, T., Akther, S., Fakhrul, A., Stanislav, C., ... & Lopatina, O. (2017). An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC neuroscience, 18(1), 1-12. PMID: 28340569 PMC5366154 DOI: 10.1186/s12868-017-0350-7
- ↑ Kim, U. H. (2014). Multiple enzymatic activities of CD38 for Ca2+ signaling messengers. Messenger, 3(1-2), 6-14. https://doi.org/10.1166/msr.2014.1030
- ↑ Kellenberger, E., Kuhn, I., Schuber, F., & Muller-Steffner, H. (2011). Flavonoids as inhibitors of human CD38. Bioorganic & medicinal chemistry letters, 21(13), 3939-3942. PMID: 21641214 DOI:10.1016/j.bmcl.2011.05.022
- ↑ Lee, D. Y., Yun, S. M., Song, M. Y., Jung, K., & Kim, E. H. (2020). Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants, 9(4), 285. PMID: 32230772 PMC7222181 DOI: 10.3390/antiox9040285
- ↑ Wang, B., Cui, S., Mao, B., Zhang, Q., Tian, F., Zhao, J., ... & Chen, W. (2022). Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-κB Signaling Pathways. Antioxidants, 11(12), 2383. PMID: 36552590 PMC9774769 DOI: 10.3390/antiox11122383
- ↑ Bashllari, R., Molonia, M. S., Muscarà, C., Speciale, A., Wilde, P. J., Saija, A., & Cimino, F. (2020). Cyanidin-3-O-glucoside protects intestinal epithelial cells from palmitate-induced lipotoxicity. Archives of Physiology and Biochemistry, 1-8. PMID: 33021853 DOI:10.1080/13813455.2020.1828480
Further reading
- Chini, C. C. S., Cordeiro, H. S., Tran, N. L. K., & Chini, E. N. (2023). NAD metabolism: Role in senescence regulation and aging. Aging Cell, e13920. PMID: 37424179 DOI: 10.1111/acel.13920
- Li, F., Wu, C. & Wang, G. (2023). Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases. Neurosci. Bull. PMID: 37253984 DOI:10.1007/s12264-023-01072-3